Table of Contents
Context: Scientists have reported in Science that addressing solid-state battery (SSB) failures might depend on applying well-established mechanical principles.
Pesky Failure in Solid-State Batteries (SSBs)
The failure was due to lithium dendrites forming on the anode and piercing the solid electrolyte, which ultimately short-circuited the battery.
Key Contributing Factors
- Metal fatigue and mechanical stress are caused by repeated charge-discharge cycles.
- Cracks and voids are forming at the anode-electrolyte interface.
- Even low charge-discharge rates caused structural fatigue, breaking the electrolyte.
Also Read: Li-Ion vs Lead Acid Batteries
What are Solid State Batteries?
- Solid-state batteries are next-generation batteries that use a solid electrolyte instead of the liquid or gel electrolyte found in conventional lithium-ion batteries.
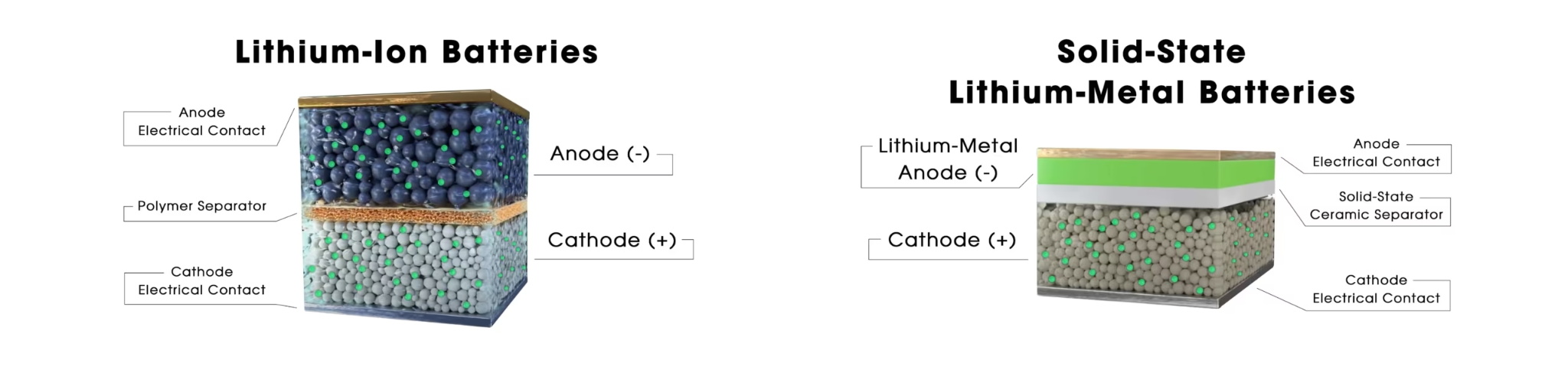
Structure and Working Principle
Components
- Anode: Often made of lithium metal, which allows for higher energy storage.
- Cathode: Made from various materials, similar to those used in lithium-ion batteries (e.g., lithium cobalt oxide, lithium nickel cobalt aluminium oxide).
- Solid Electrolyte: The key differentiator, made from ceramics, glass, sulfides, or solid polymers, separating the anode and cathode and allowing only ions to pass through.
Operation
- During discharge, lithium ions move from the anode to the cathode through the solid electrolyte, while electrons flow through the external circuit, powering the device.
- During charging, the process reverses: lithium ions travel back from the cathode to the anode
Advantages
- Higher energy density → Longer range for electric vehicles.
- Faster charging → E.g., 15-minute fast charge capability.
- Improved safety → Eliminates risk of leaks and thermal runaway.
- Compact design → Enables smaller and lighter battery packs.

 Advanced Air Defence Radars: Types, Comp...
Advanced Air Defence Radars: Types, Comp...
 Ion Chromatography, Working and Applicat...
Ion Chromatography, Working and Applicat...
 Broadly Neutralising Antibodies (bNAbs):...
Broadly Neutralising Antibodies (bNAbs):...